Corrosion, Degradation and Oxidation
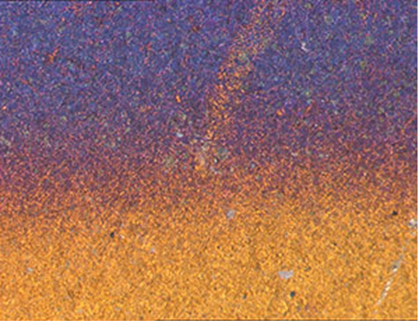
 Corrosion, oxidation and degradation commonly spoil the aesthetics, leads to component weakening, the requirement for remedial actions and, in the worst case, failure of materials in products. Often this is directly due to the aggressive environment that a product is exposed to over its operational life. In the case of plastics or paint coatings it can be embrittlement or discolouration, paint delamination or solvent swelling. Metals can be attacked by aqueous based corrosion,oxidised at elevated temperature or stress corrosion cracking.
Corrosion, oxidation and degradation commonly spoil the aesthetics, leads to component weakening, the requirement for remedial actions and, in the worst case, failure of materials in products. Often this is directly due to the aggressive environment that a product is exposed to over its operational life. In the case of plastics or paint coatings it can be embrittlement or discolouration, paint delamination or solvent swelling. Metals can be attacked by aqueous based corrosion,oxidised at elevated temperature or stress corrosion cracking.
In order for aqueous corrosion to occur the following have to be present:-
- Anodic area where components degradation occurs.
- Cathodic area, the driving force for the corrosion process.
- Electrolyte to transport corrosive species and various ions.
- Electron conduction path connecting anode and cathode.
 The corrosion rate is dependent on many factors, some of which are listed below:-
The corrosion rate is dependent on many factors, some of which are listed below:-
- Relative areas of the anode and cathode.
- Electrolyte conductivity and whether the metal is immersed in water, underground or just corroding as a result of surface moisture related to the humidity.
- Availability of fresh reactants; corrosive species such as oxygen and chloride ions, protons (H+).
- Removal of corrosion products and the tendency of the oxide to spall away which allows attack to continue virtually unhindered
- Resistance in the metallic circuit
- Electrical Resistance and durability of any protective coatings or surface treatments
- Temperature and pH.
Mechanisms to minimise the impact of the environmental attack are often in place, but have failed to be effective for a range of possible reasons:
- Unusual or unexpected exposure conditions (chemical, humidity, pH, turbulence from flowing water etc, differential aeration etc.).
- Mistaken use or wrong choice of material or metal alloy for the environment.
- Galvanic cell set up with another nearby metal component electrically in contact with the corroding part, with the circuit completed by an electrolyte, which can be as little as surface adsorbed water.
- Failed protection system.
- Ineffective or insufficient biocides or inhibitors in a closed system like a heating system as microbiological / microbial attack can be very aggressive.
 The protection system designed to slow down or prevent corrosion can be
The protection system designed to slow down or prevent corrosion can be
- Passivation having or deliberately growing a protective oxide. Stainless steel has a protective surface oxide and aluminium and titanium are often anodised to grow thicker protective oxides.
- Choice of material / alloy to insure the material is passive or immune in the environmental conditions the component is likely to see over life.
- Avoid two phase alloys which can provide the anode and cathode locally close together and give phenomena such as dezincification of brasses where one phase can be preferentially corroded away.
- Cathodic protection by use of a sacrificial anode or impressed current to make a key component cathodic and so not corrode.
- Anodic protection using impressed current to make a material passive.
- Painting, spraying or dip coating to provide a diffusion barrier to slow down or stop the ingress of corrosive species such as oxygen or chloride ions.
- Use of conversion coatings.
- Avoid rough surfaces as points protruding into the electrolyte will preferentially corrode.
- Cracks or small pits should be avoided as, from the catchment area principle, the anode at the crack tip (because of a lower availability of oxygen) is small with respect to the cathode and attack can be relatively fast giving pits. Aluminium and stainless steels or alloys with passive filme are susceptible to pitting corrosion.
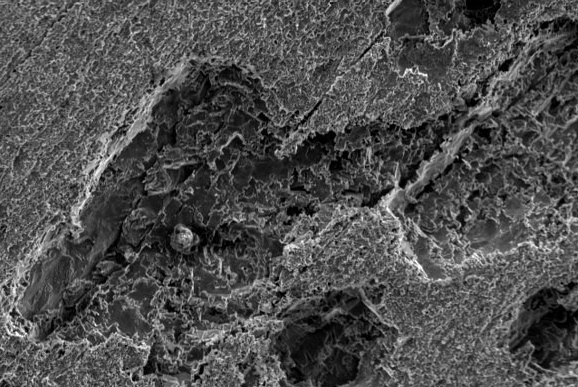
Depending on the materials involved SEM/EDX and optical microscopy together with a combination of various surface analysis techniques, Ion Chromatography and FTIR generally are used to analyse and identify the failure mechanism and then the source of the aqueous corrosion. This can lead to the laboratory making recommendations on how to best avoid or solve the problem in the future.
Application Notes